In his first year, President Donald Trump should develop a charter for, assign priorities to, and establish by executive order a new presidential commission on bioethics. Even though its membership should be diverse in background, experience, and expertise, it is reasonable for the president to set up a commission that is generally congenial to his administration’s values. The commission’s main function will be to provide advice and counsel to the president. It can also report directly to federal departments and agencies and to congressional committees. Further, its meetings and reports can contribute to public discourse and education about bioethics.
Doing so will build on the best practices of previous presidents, who have used such outside guidance to address complex ethical challenges in the biological sciences, medicine, and health care. The new president can choose from several options in how to design such a commission, and which topics to address. But having such a commission will ensure that thoughtful examination will guide emerging technologies.
While bioethics, a term coined in 1970, has various meanings, a primary definition features ethical reflection and judgment regarding the biological sciences, medicine, and health care. Bioethical reflection and judgment often focus directly on such activities as clinical care, but public bioethics is also vitally important. It focuses on public policies, many of which fall under the auspices of the executive branch, particularly the office of the president of the United States, who may direct departments and agencies to consider and develop policies on bioethical topics. Many of these initiatives will also involve interactions with Congress and the courts. In addition to public policies, the president may also have a significant impact on public discourse and education in bioethics, which may in turn shape public policies.

In the event of a public health crisis such as a Ebola pandemic, the president should develop ethically acceptable public health policies and practices.
The president’s priorities should include ensuring the retention and implementation of recently updated rules and guidelines for protecting human research participants and, at the same time, facilitating important research; developing a framework for ethically acceptable public health policies and practices in the event of public health crises such as pandemics of Ebola and the Zika virus; and moving forward the still evolving societal and public policy discussion about human gene editing. Each of these subjects involves questions about how best to protect individual liberties while promoting the common good. In addition, other emergent ethical problems, occasioned by scientific and technological breakthroughs, can be helpfully addressed by a presidential bioethics commission just as the advent of human cloning, human embryonic stem cell research, and synthetic biology required the attention of one or more of the last three commissions.
Presidential commission on bioethics
Many presidential actions have implications for bioethical controversies. For instance, Trump’s nomination of federal appellate judge Neil Gorsuch for appointment to the U.S. Supreme Court has implications for such “hot button” bioethical issues as abortion and religious liberties not to provide contraception. Several presidential actions in public bioethics, particularly under the circumstances previously noted, can benefit from a systematic and sustained examination by an interdisciplinary body. For most of the last 40 or so years presidential (or congressional) commissions have shouldered much of this task.

There are three main types of institutional structures for public bioethics: a standing national commission, a series of commissions, or funding nongovernmental committees.
There are three main types of institutional structures for public bioethics, each with distinct advantages and disadvantages. The first is a standing national commission, with a broad mandate and the opportunity to identify specific areas for attention as well as to respond to presidential requests. A second is a series of commissions with mandates that are more focused, targeted, and limited, having to do with particular subjects, such as organ transplantation, human fetal tissue transplantation research, or human embryo research. A major advantage of a standing commission over a series of subject-limited commissions is that it has time to mature as a deliberative body, to develop practices of collective analysis and reasoning, to be prepared for new challenges, and so forth. A major advantage of a targeted, focused commission is that its members can be selected on the basis of their potential contributions to the specific task at hand, which the committee can pursue with concentrated attention and effort. However, it will still take some time to get each ad hoc committee up and running.
A third possibility is to fund nongovernmental committees to produce reports and make recommendations for the president and other governmental bodies. The specific studies undertaken by committees established by the National Academies of Science, Engineering, and Medicine provide one such model. Often established in response to requests and with support from federal departments or agencies such as the Food and Drug Administration (FDA) or the National Institutes of Health (NIH), these independent committees prepare consensus reports, often on single issues such as, recently, novel techniques for prevention of maternal transmission of mitochondrial DNA diseases. Such committees may appear to be less “political” than a presidential or a congressional commission. However, they have a distinct disadvantage: even though they may include public members as well as experts, their process of developing findings, conclusions, and recommendations is not as open as that of governmental bioethics commissions. They may appear more objective and impartial, but they are less publically transparent.

After Dolly the cloned sheep was born, President Bill Clinton asked the National Bioethics Advisory Commission to prepare a report recommending federal policies on human reproductive cloning.
Each of these three institutional mechanisms can contribute significantly to presidential and other governmental bioethical policy making. Which one is best for a particular topic depends in part on process values such as transparency and efficiency. While not every bioethical topic needs to be addressed by a standing presidential bioethics commission, such a commission has the substantial advantage of being able to address a range of bioethical topics, sometimes interrelated, in public over time and the advantage also of being ready to address fairly quickly new issues when they emerge. For instance, following the announcement of the cloned sheep Dolly’s birth, President Bill Clinton gave his relatively young National Bioethics Advisory Commission 90 days to prepare a report recommending possible federal policies toward human reproductive cloning.
Setting priorities for public bioethics
At this point there are several bioethical topics that the new president—and in some cases a new presidential bioethics commission—should take under advisement. Of the three noted below, one (human gene editing) will require special attention because it unsettles long-dominant bioethical and public policy paradigms.
Research involving human subjects or participants
One of the central areas of public bioethics is research involving human “subjects,” now increasingly called “participants” to emphasize that these individuals collaborate with researchers on behalf of the society and that their (or their surrogate’s) voluntary, informed consent is indispensable. Research involving human participants was the focus of the very first commission, the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1974–78), which was established in response to several scandals in biomedical research. The most notorious scandal was the U.S. Public Health Service’s research for over 40 years on the effects of untreated syphilis on close to 400 African American males in and around Tuskegee, Alabama. The commission’s work led to new regulations to govern federally funded research (and much other research). Other subsequent commissions have reexamined these rules, in part because research involving human participants has changed so much in volume, the number of institutions and researchers involved, international scope, the nature of the research (e.g., involving genetic information), complexity, etc. Now, after several decades, some of the extant rules may underprotect while others overprotect human participants and some make it difficult or burdensome to conduct needed research.
On January 19, 2017, the newly revised Common Rule—the set of federal regulations for ethically conducting research involving human subjects—was published in the Federal Register, following discussions that had germinated since its original enactment in 1991, deliberations that started in earnest in 2011, and broad input throughout the extended process of rulemaking. The new rule, which is scheduled to take effect in 2018, might appear, at first glance, to be a good target for revocation given the new administration’s commitment to extensive deregulation. However, this revision of a long-established rule is designed to facilitate valuable research, in part by reducing unnecessary regulatory burdens, for instance, by generally requiring a single Institutional Review Board review in multi-institutional studies and by standing against the needlessly long and complex consent forms. At the same time, it is designed to better protect the rights and welfare of research participants. It would be a mistake to rescind this revised Common Rule without a thorough review through one of the three mechanisms of public bioethics described above.
An ethical framework for public health
In an era of globalization, marked by fast and easy travel, serious threats from infectious diseases confront the world. Ebola and the Zika virus are just two recent examples, and the World Health Organization has identified a number of other potentially serious threats. The new presidential commission should build on the work on public health ethics (developed in the context of Ebola) by the (current) Presidential Commission for the Study of Bioethical Issues, and on earlier work by the Ethics Subcommittee of the Advisory Committee to the Director of the Centers for Disease Control and Prevention (CDC). This is particularly important not only because of the recurrent threat of epidemics and pandemics but also because in 2013 the Advisory Committee voted to terminate its Ethics Subcommittee and to set up ethics workgroups on an ad hoc basis. In view of society’s tendency to lurch from one crisis to another, the new presidential commission could and should attend more systematically to several critical issues in public health to create a clear and practical framework to guide policy. These issues include reduction of disparities in population health in outbreaks of infectious diseases; the fair and equitable distribution of scarce goods, such as vaccines, in a crisis; and the conditions for justifiable restrictions of liberties, for instance, through quarantine and isolation. This could enable the president to take the lead in developing a policy framework to address public health crises, which require federal and state action.
Human gene editing
CRISPR is a kind of molecular scissors or nanoscissors that is more precise than its predecessors for gene editing.
Long the subject of science fiction, human genetic engineering in the form of gene editing is now emerging as a realistic possibility thanks to major scientific and technological breakthroughs. One such technology, CRISPR/Cas9, is a kind of molecular scissors or nanoscissors that is both easier to use and more precise than its predecessors. It is being readied for phase I cancer trials involving human participants in the U.S. and in China. Other new gene editing technologies are already in early clinical trials.
For several decades, two supposedly bright lines have delimited policies and practices of human genetic modification. The first line divides somatic gene modification, which involves cells that do not pass on genetic modifications to offspring, from germ-line modification, which involves cells that pass on genetic modifications to offspring and future generations. The second line divides gene therapy (i.e., treatment or prevention of a disease, such as cancer) from genetic enhancement (i.e., improving nondiseased capabilities, such as intelligence or height). While these lines are not as bright as commonly supposed, they remain important.
Versions of the following figure been widely used in efforts to depict these lines:
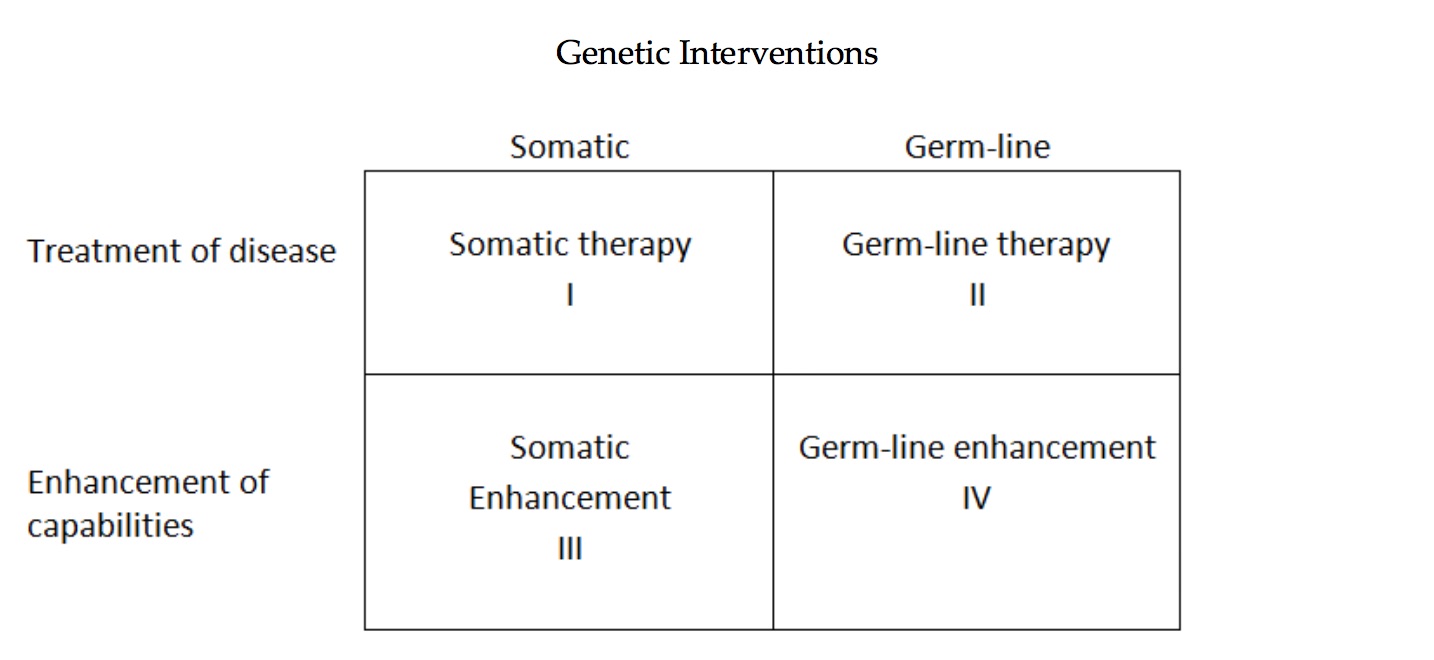
These distinctions became embedded in public policy, for example, in the guidelines of the NIH’s Recombinant DNA Advisory Committee (RAC), especially to assure the public that it would be possible to move forward with “human gene therapy” trials without legitimating either germ-line interventions or genetic enhancements that are considered ethically problematic. An intervention in Category I (above) has generally been deemed acceptable, if it is determined to be safe for the recipient, because it is similar to other medical treatments intended to provide a medical benefit to the individual patient (such as a bone marrow transplant for leukemia).
Interventions in Category II cross the line into germ-line modification—for instance, genetic modifications of embryos are heritable and can thus be passed on to future generations. Pressure will almost certainly emerge in the near future to move to germ-line interventions because, in some cases, this may be the most effective and efficient way to treat/prevent some diseases and because those same treatment/prevention benefits will be passed on to future offspring. However, major concerns immediately arise. One has to do with the experimental genetic modification of embryos. Another concern focuses on the unquantifiable risk to future generations. Still another concern is that future generations cannot consent to these risks, which are imposed on them and which may be irreversible. This can even be considered interference with evolution. Whereas proponents of interventions in Category II sometimes insist we should seize this power and speed up the process of evolution, critics respond that we lack the capacity to reliably predict and control these changes and their effects as well as the ethical wisdom to assess them. Finally, concerns arise about the possible negative impact of these genetic modifications on attitudes and practices toward persons with disabilities. And simmering eugenic forces should not be neglected.
Even though we can clearly locate some interventions on the side of treatment/prevention and others on the side of enhancement, the line is not always easy to draw. And, while there is substantial opposition to enhancement in either somatic or germ-line interventions, it is much greater in the latter, as expressed in worries about “designer babies.” Social justice concerns arise for both somatic and germ-line enhancement: the “haves” would benefit more than the “have nots,” further deepening social inequalities. Such concerns figure in public opinion surveys. For instance, a Pew Research Center report in 2016 found that the U.S. public is wary of biomedical technologies to enhance human abilities and that their fears and concerns outweigh their hopes.
A common underlying concern is that of hubris, often critically expressed in the metaphor of “playing God.” Still another metaphor—the slippery slope—is noteworthy as we think about the implications of these and other genetic interventions. An apparently innocuous first step may produce consequences that we cannot control, perhaps because of inadequate institutional mechanisms. Even if we could assume a strong scientific and public consensus against genetic enhancements, the U.S. regulatory system would probably not effectively prevent them. For instance, even if the FDA approves a gene transfer intervention as treatment for a specific medical condition or disease, this same intervention could also be used for enhancement purposes absent further regulation.
Finally, the new president and his administration will have to consider the governance of gene editing, both domestically and globally. Of course, governance is not limited to governments and their policies. It encompasses various other social institutions as well, and its exercise is not limited to laws and regulations but involves professional standards and the like. Nevertheless, there is a central role for government, and the U.S. government needs to continue to play a pivotal, though nonexclusive, role in the still nascent complex global governance of gene editing, as discussed, for instance, in the 2015 International Summit on Human Gene Editing. The U.S. government’s laws and regulations, as well as its investment decisions, will be crucial both tangibly and symbolically.
Given the perennial problems in health care and in research involving human participants, the outbreaks of serious and even deadly infectious diseases, and the rapid pace of global scientific and technological change, the new president will surely confront and need to address several bioethical problems. How the president meets these challenges and helps our government, the scientific and biomedical communities, and the public deliberate about appropriate policies and practices in response will be one of the tests—and measures—of our nation’s new chief executive.